

Graphene is the fundamental building block of graphitic mediums. It is composed entirely of carbon atoms, and 1mm of graphite comprises approximately 3 million graphene sheets. The carbon atoms are perfectly arranged in a hexagonal honeycomb formation just 0.3 nanometers thick, with 0.1 nanometers between each particle.
Nanographene is a component of graphene. The nanographene production is through dehydration, where there is the selective removal of hydrogen atoms from organic molecules of hydrogen and carbon. It has more complex fabrication procedures compared to generic graphene.

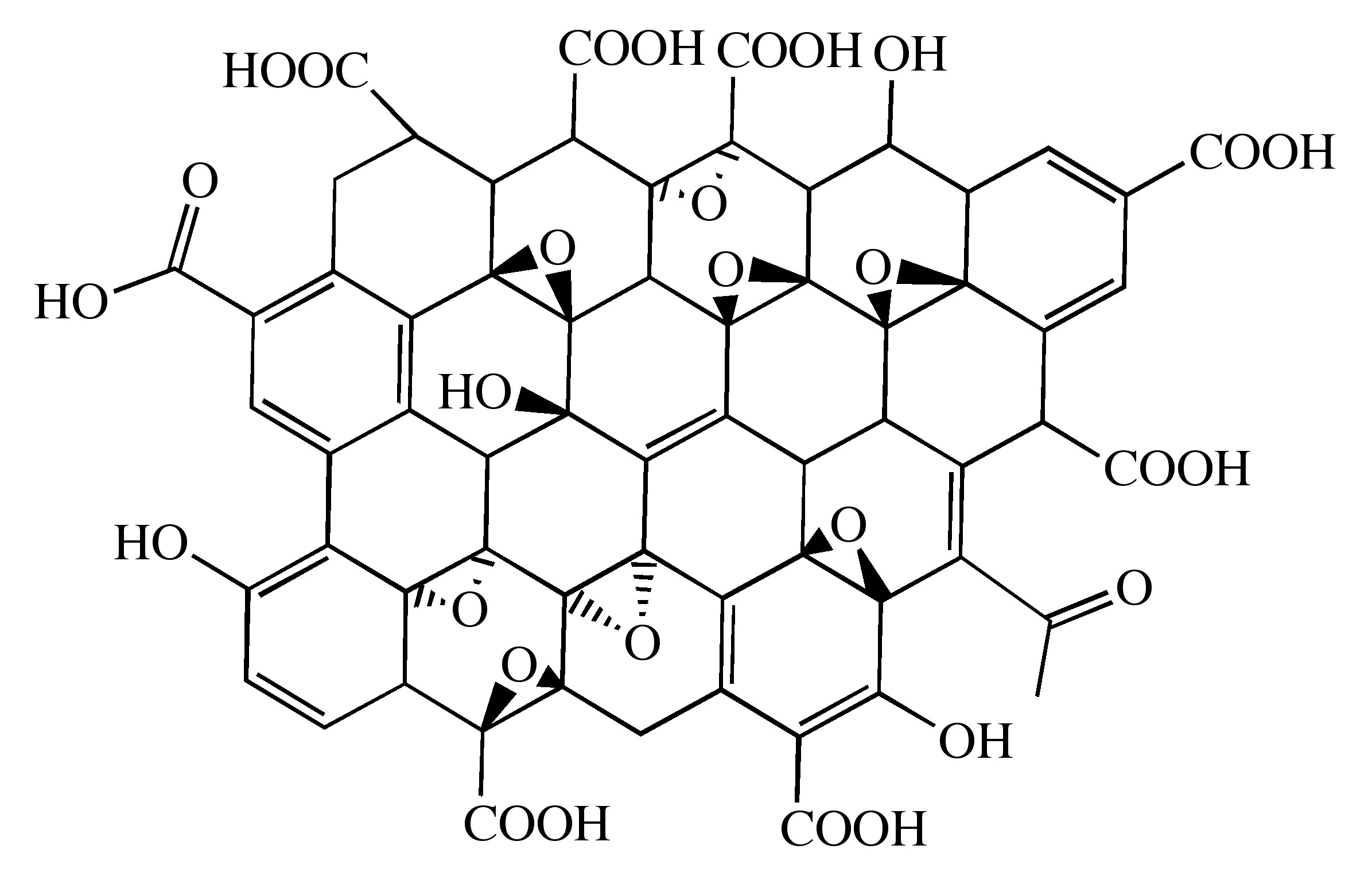
Technically, graphene is non-metal. However, scientists often consider it a quasi-metal because of its properties, such as being a semiconducting material. Graphene has many distinctive attributes that other non-metallic materials do not have. Graphene is a two-dimensional planar layer of carbon that occurs as an allotrope of carbon. Each carbon atom in a hexagonal array is covalently bonded to three other carbon atoms, leaving one free electron per carbon atom. This free electron resides in a p-orbital above the materials plane, enabling the effective transmission of electricityWith the hexagonal lattice formation of graphene, the structure's holes enable phonons to travel through unhindered, and allows a high thermal conductivity. As compared to usual materials, graphene also has unique properties that make it ideal for electronic applications.
Graphene is a material with genuinely exceptional properties. Together with the amount of carbon in nature, these properties have made graphene a highly researched material with various applications. Graphene's most notable properties are:
Graphene is one of the best electrical conductors in the world. The carbon’s unique atomic formation in graphene enables electrons to pass through high velocity without scattering, saving valuable wasted energy in other conductors.
Durability and rigidity are one of the properties that make graphene an individual material and a strengthening composite material. The hexagonal lattice of graphene that forms sp2 bonds creates the stability of graphene.
The most robust material on earth is single-layer graphene. It is because of its strength of 42 N m-1 that it is equivalent to an intrinsic strength of 130 GPa (gigapascal).
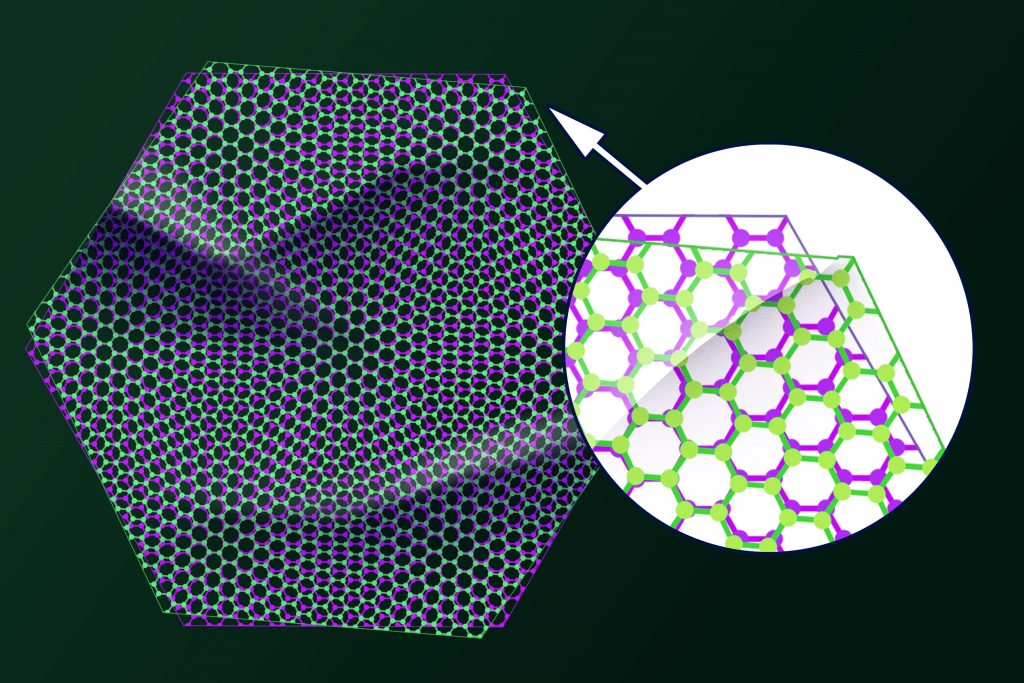
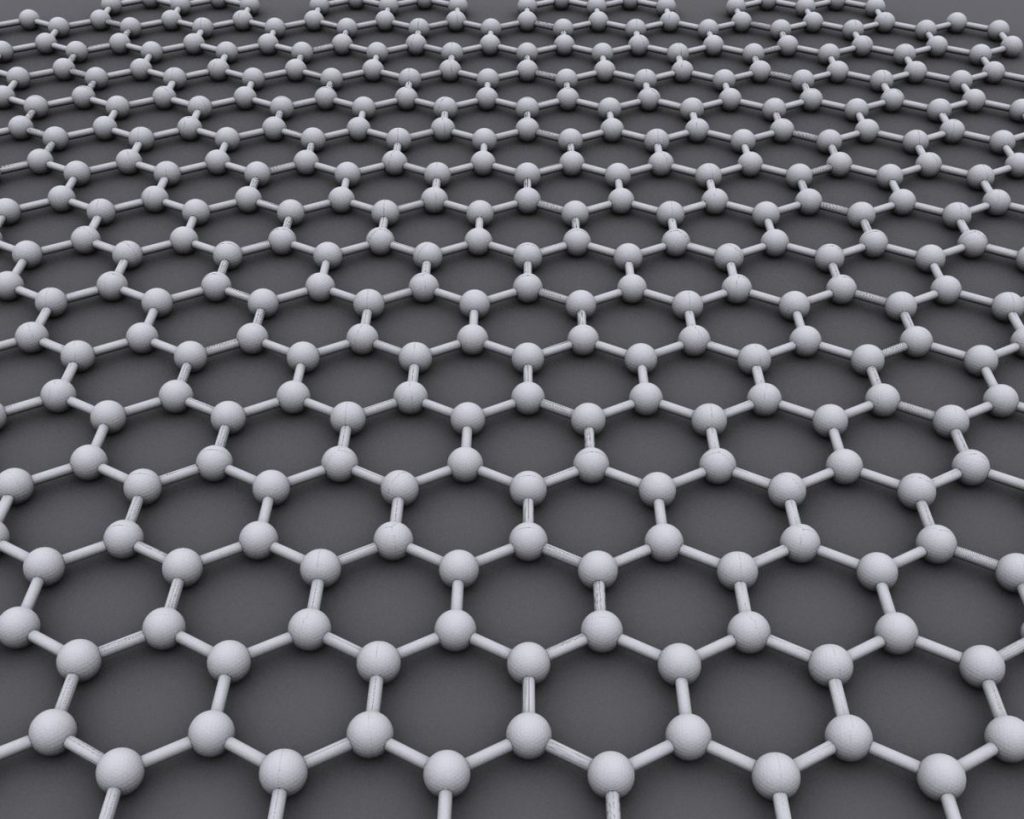
The graphene’s critical stress intensity factor is 4.0 ±0.6 MPa (megapascal), making its toughness one of the essential mechanical properties. This attribute applies to engineering applications.
Graphene is indeed a wonder material, assuring the world of innovations. Some of the technologies enhanced with graphene are the following:

Recently, researchers developed capacitive deionization (CDI) technology. As electrodes for capacitive deionisation, CDI uses graphene-like nanoflakes. According to the results, the performance of CDI is better compared to traditional activated carbon materials.
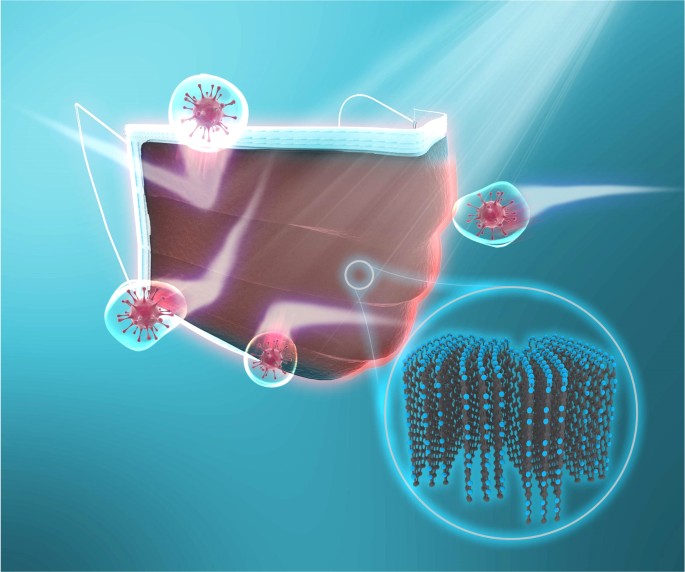
During the pandemic, the need for face masks has increased. In recent research, the team favourably produced graphene-enhanced face masks. This mask comes with an 80% anti-bacterial efficiency.
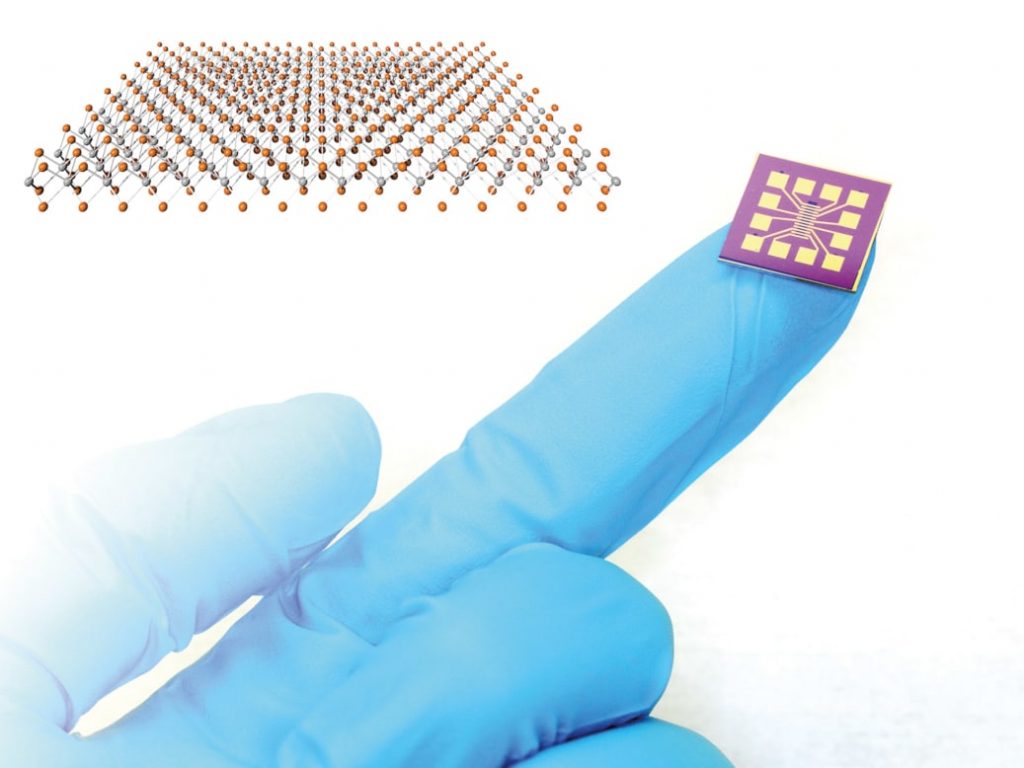
The graphene-enhanced sensor can recognise 60-second changes in its neighbouring environment. Graphene’s layer has a consistent and planar formation of atoms. Each atom within the sheet exposes itself to the surrounding environment. It enables graphene to identify changes in its surroundings at micrometre scales, resulting in an excellent sensitivity level.
The use of graphene in functional inks is to provide anti-corrosion and heat purposes. By combining graphene into ink formulations, the influence of graphene’s conductivity properties can cause inks to become more conductive.


Incorporating both the anode or the cathode in different battery systems can increase the battery’s efficiency. Aside from that, graphene on the battery will also enhance the charge/discharge cycle rate.
Because of its adaptable nature, the successful incorporation of graphene in various technological variations is available on the market today. Thanks to graphene, we can use more efficient technologies. With continuous research around the world, it is possible to develop more advanced technologies in the future.
For the past decades, there has been an infinite quantity of discoveries and developments. Aside from bronze, iron, silicon, and aluminum, graphene is one of these innovations. It continuously contributes to several designs today that embody the advancement of the world.
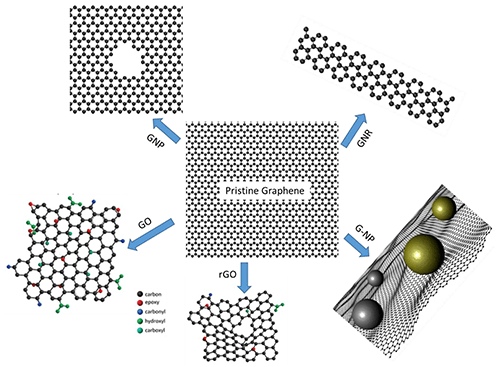
As reported by the International Union of Pure and Applied Chemistry (IUPAC), graphene is a terminology used in terms of structural association, reactions, or other attributes of individual sheets of carbon. Graphene is reciprocating polycyclic aromatic hydrocarbon rings of six carbon atoms. Graphene serves as the foundation form of the following graphitic structures of carbon:
Fill the form below: